What are clinical trials?
Clinical trials are the final step in years of research for new treatments for blood cancer.
The aim of clinical trials is to find new treatments that will result in better outcomes for patients and a better quality of life, with less side effects.
In 2020, Cure Leukaemia committed to funding the Trials and Acceleration Programme (TAP) for 3 years, which connects patients with potentially life-saving clinical trials through 12 centres and 12 specialist nurses across the UK.
In 2023, we were thrilled to announce that we were able to expand the TAP network to now cover 15 centres across the UK.
Clinical Trials in the UK
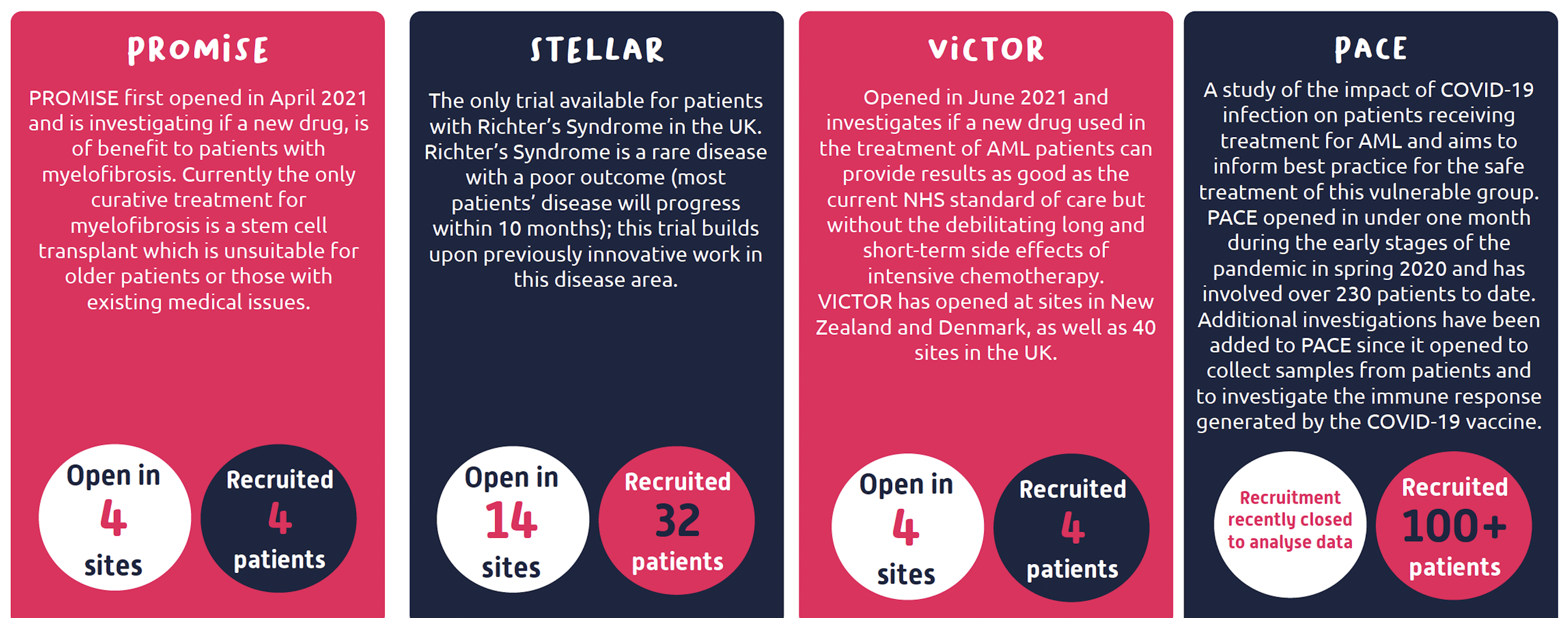
The major issue is that pharmaceutical companies are creating new potentially life-saving drugs at a faster rate than they can currently be tested. Without appropriate clinical trials, and specialist nurses to deliver them, these trials cannot run.
Some of these drugs will never be tested and will simply gather dust behind locked doors. Patients will keep dying of blood cancers whilst the magic bullet to treat their disease is sitting on a scientist’s bench.
Cure Leukaemia are proud to fund 15 blood cancer centres across the UK to form the Trials Acceleration Programme (TAP) network.
Since the TAP Network was formed

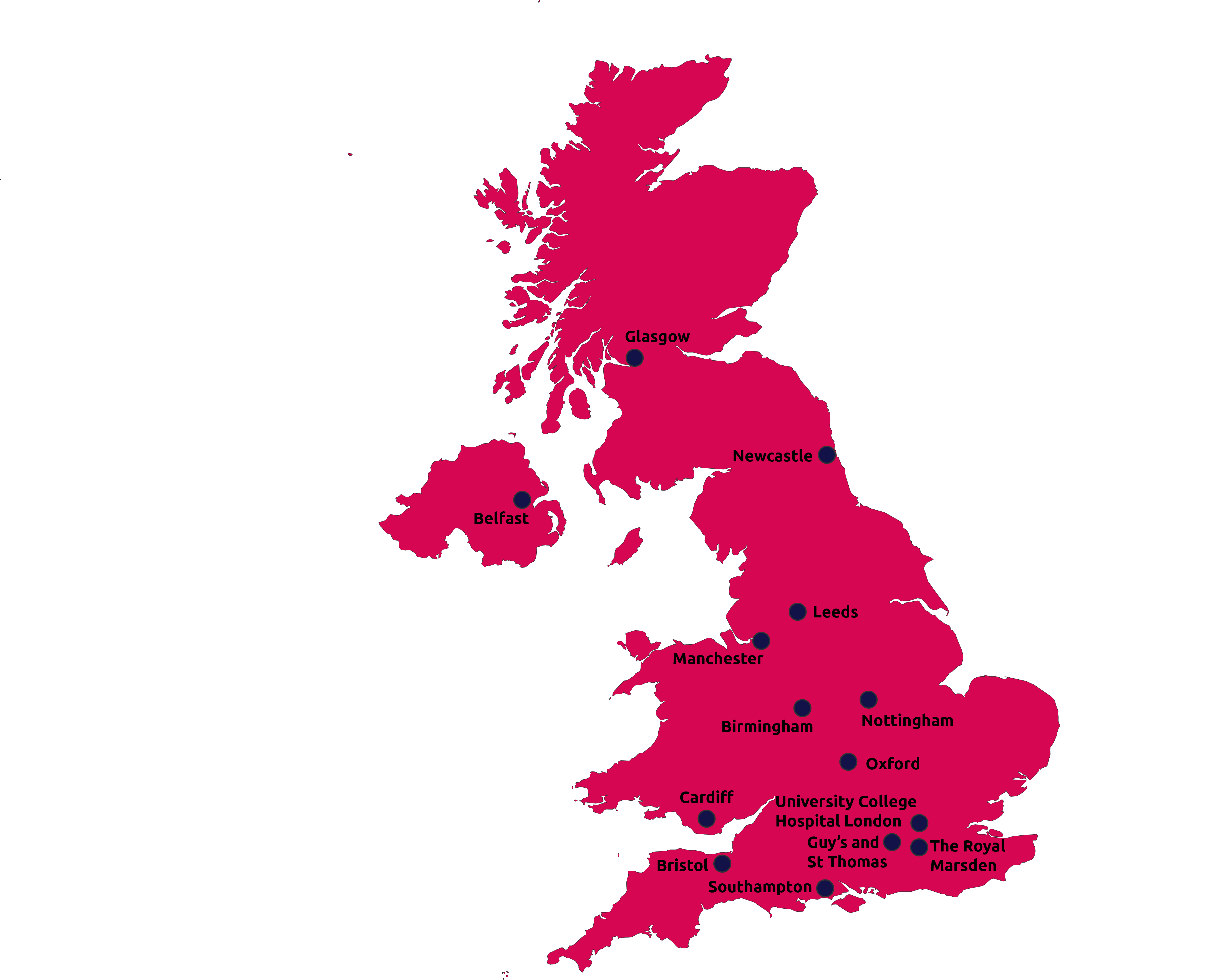
The TAP Network
The TAP Network consists of 15 centres across the UK in all major cities.
Cure Leukaemia funds dedicated Research Nurses at all 15 of these centres, who specialise in recruiting patients for pioneering trials.
Find out more about Cure Leukaemia funded research nurses, and the role they play in clinical trials and the TAP Centres.

